Abstract
Coagulation factor XII (FXII) has attracted significant interest as a potentially transformative antithrombotic drug target, with preclinical models consistently showing that FXII deletion or blockade protects against thrombosis without causing hemorrhage. A long-standing observation has been that stimulated platelets can support activation of FXII, thereby linking platelet function to FXIIa generation and thrombus formation. However, the mechanism by which FXII is recruited and activated at the platelet surface remains unclear and a source of considerable controversy.
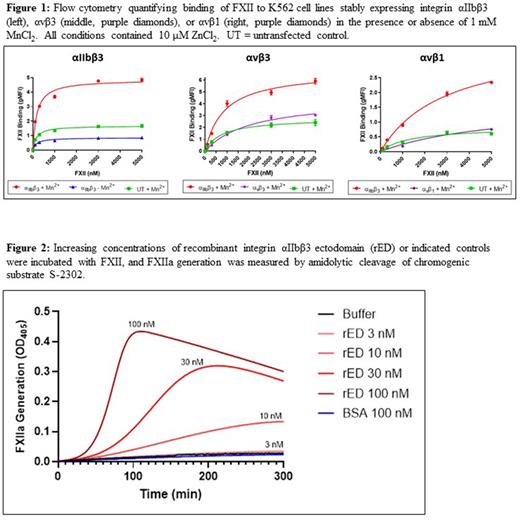
In order to explore the FXII-platelet interaction, we performed a mass spectrometry-based proteomic screen of potential FXII binding partners in the solubilized membrane fraction of SFLLRN-activated platelets. FXII co-immunoprecipitated with integrins αIIb and β3, with strong signals also observed for several integrin-associated proteins. These results were corroborated by Western blot of FXII co-immunoprecipitation with washed stimulated platelets and pulldown of FXII from dilute human plasma by beads coated with activated integrin αIIbβ3. Using inactive integrin from resting platelet lysate, we found that only by inducing the activated conformation of αIIbβ3 with MnCl2 could the receptor bind FXII. When flow cytometry was performed on K562 cell lines stably transfected with integrin αIIbβ3, αvβ3, or αvβ1, only those expressing αIIbβ3 in the presence of MnCl2 supported binding of FXII-FITC (Figure 1).
We next evaluated the need for αIIbβ3 to support platelet-dependent FXIIa generation. Platelets from integrin β3 knockout mice (ITGB3-/-) demonstrated significantly decreased binding to FXII-FITC by flow cytometry, and ITGB3-/- platelet-rich plasma (PRP) stimulated with PAR4 agonist peptide failed to generate FXIIa in a chromogenic assay compared to PRP from wild-type littermate controls. Similarly, platelets from a patient with congenital biallelic loss of integrin αIIb (Glanzmann's thrombasthenia) also failed to bind or activate FXII compared to platelets from healthy donors. To further interrogate the role of αIIbβ3 in FXII activation, we recombinantly generated the soluble αIIbβ3 ectodomain (rED). Bio-layer interferometry (Octet) steady state analysis showed that FXII bound the rED with KD=16 nM. We found that rED greatly enhanced the rate of FXIIa generation in solution (Figure 2), with an increase of 133-fold in Kcat/Km and 203-fold in Vmax measured for FXII catalytic activity. Further, when rED was added to FXII in the presence of factor XI (FXI), we found a significant enhancement in FXIIa-dependent activation of FXI.
All known physiologic ligands of αIIbβ3 contain an RGD motif, which is required for binding to the integrin. However, FXII does not have an RGD or equivalent sequence. We sought to better understand the potentially non-canonical nature of FXII-αIIbβ3 binding. We found that PAC-1 antibody, which blocks RGD-dependent macromolecular ligand binding to αIIbβ3, inhibited binding of fibrinogen-FITC to activated human platelets but failed to block FXII-FITC binding. We next studied the impact of two αIIbβ3 inhibitors on the FXII-integrin interaction. Eptifibatide blocks RGD-dependent ligand binding to αIIbβ3 and induces a large structural change in the receptor, causing it to assume an "extended open" conformation. By contrast, antibody 10E5 blocks ligand binding without inducing a conformational change in αIIbβ3. In a solid-phase assay, 10E5 did not inhibit binding of FXII to αIIbβ3, whereas eptifibatide partially blocked binding, consistent with an allosteric effect mediated by the transition to the extended open conformation. Likewise, eptifibatide partially inhibited FXIIa generation by stimulated PRP and by purified αIIbβ3, while 10E5 had no effect in either assay.
Our data are consistent with a model in which non-canonical binding of FXII to integrin αIIbβ3 is responsible for FXII activation at the platelet surface. Future studies will be directed at dissecting the molecular mechanism of FXII activation by αIIbβ3 and determining whether non-RGD dependent binding may generalize to other ligands of the integrin. Taken together, our work contributes to a novel understanding of pathologic FXIIa generation during platelet activation and thrombus formation.
Disclosures
Bendapudi:Alexion: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal